Global Pharma Clinical Trial Digitization Market - Size, Share, Demand, Industry Trends and Opportunities
Global Pharma Clinical Trial Digitization Market, By Services (Drug Dose Adjustment, Drug Impact Monitoring, Medical Prescription System, Bioprinting, Preventive Therapy, Individualized Drug Printing), Application (Clinical Data Management, Trial Monitoring, Patient Recruitment and Enrollment), Themes (Digital Continuity Across Clinical Trial IT Systems, Patient-centric Remote and Virtual Trial Design, Direct-to-patient Home Services) Country (U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E, Egypt, Israel, Rest of Middle East and Africa) Industry Trends
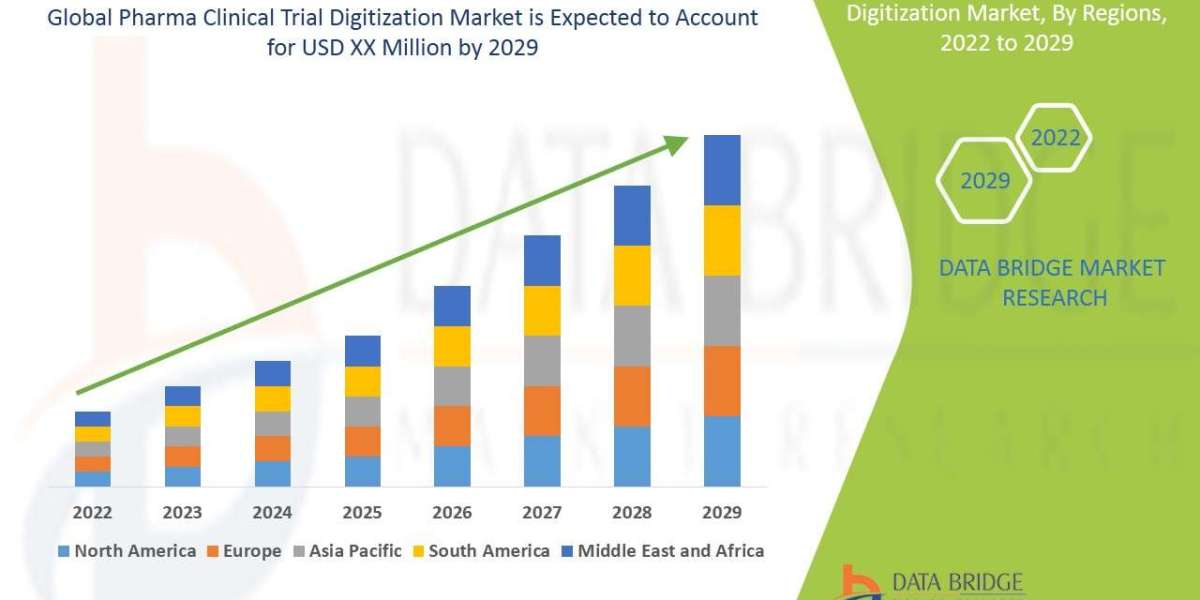
Data Bridge Market Research analyses that the pharma clinical trial digitization market to account growing at a CAGR of 6.2% in the forecast period of 2022-2029. The growing demand for personalised medicine is expected to open up new opportunities for the pharmaceutical clinical trial digitization market.
Access Full 350 Pages PDF Report @
https://www.databridgemarketresearch.com/reports/global-pharma-clinical-trial-digitization-market
**Segments**
- Based on type, the Pharma Clinical Trial Digitization Market can be segmented into Software and Services. The software segment includes solutions for electronic data capture, clinical trial management systems, randomization and trial supply management, electronic patient-reported outcomes, and others. On the other hand, services encompass consulting, implementation, support, and maintenance services that aid in the digital transformation of clinical trials.
- Geographically, the market can be divided into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America has traditionally been a dominant market due to the presence of key pharmaceutical companies, advanced healthcare infrastructure, and government initiatives to promote digitization in clinical trials. However, the Asia-Pacific region is witnessing significant growth attributed to improving healthcare facilities, increasing R&D investments, and a large patient pool for clinical trials.
**Market Players**
- Some of the key players in the Pharma Clinical Trial Digitization Market include Oracle Corporation, Parexel International Corporation, Medidata Solutions, Inc., PRA Health Sciences, Inc., and IQVIA. These companies are at the forefront of driving innovation in digital technologies for clinical trials. Oracle provides a comprehensive suite of software solutions for efficient data management and analysis, while Medidata offers cloud-based platforms for end-to-end clinical trial management. PRA Health Sciences specializes in conducting complex clinical trials globally, and IQVIA is known for its advanced analytics and real-world evidence solutions.
- Additionally, emerging players such as CliniOps, Inc., Phlexglobal Limited, and Veeva Systems are making significant contributions to the market with their focus on streamlining clinical trial processes through digital technologies. These companies offer specialized services like document management, regulatory compliance, and integrated data platforms to enhance the efficiency and effectiveness of clinical trials in the pharmaceutical industry.
https://www.databridgemarketresearch.com/reports/global-pharma-clinical-trial-digitization-marketPharma clinical trial digitization is rapidly transforming the landscape of the pharmaceutical industry, offering a wide range of benefits such as improved efficiency, enhanced data accuracy, cost savings, and accelerated decision-making. The adoption of digital technologies in clinical trials has become imperative for pharmaceutical companies to remain competitive in an increasingly complex and fast-paced environment. By digitizing various aspects of the clinical trial process, such as data capture, patient monitoring, and regulatory compliance, organizations can streamline operations, reduce manual errors, and ultimately bring innovative therapies to market more quickly.
One of the key drivers of the pharma clinical trial digitization market is the increasing complexity of clinical research, driven by the growing demand for personalized medicine and the need to address rare diseases. Digital tools play a crucial role in managing the vast amounts of data generated during clinical trials, enabling researchers to analyze information more efficiently and derive actionable insights. Moreover, the shift towards decentralized clinical trials, prompted by the COVID-19 pandemic, has further accelerated the adoption of digital technologies to facilitate remote monitoring, telemedicine, and virtual patient engagement.
In addition to enhancing operational efficiencies, pharma clinical trial digitization also presents opportunities for improving patient engagement and retention. By leveraging mobile apps, wearables, and other digital health tools, pharmaceutical companies can create a more patient-centric approach to clinical trials, leading to better recruitment rates, higher participant satisfaction, and ultimately, more reliable study outcomes. Furthermore, digital technologies enable real-time data collection and analysis, allowing researchers to adapt protocols dynamically, monitor patient safety more effectively, and make informed decisions throughout the trial lifecycle.
As the market for pharma clinical trial digitization continues to evolve, industry players are investing in cutting-edge technologies such as artificial intelligence, machine learning, blockchain, and IoT to further enhance the efficiency and reliability of clinical research. These advancements enable predictive analytics, personalized medicine approaches, and risk-based monitoring strategies that have the potential to revolutionize the drug development process. Additionally, partnerships and collaboration between pharmaceutical companies, technology providers, regulatory**Global Pharma Clinical Trial Digitization Market Analysis**
- The global pharma clinical trial digitization market is experiencing significant growth driven by the increasing complexity of clinical research, demand for personalized medicine, and the shift towards decentralized trials post-COVID-19. Digital technologies are essential in managing data, enabling efficient analysis, and supporting innovative therapies' development.
- The market is segmented by type into Software and Services, with software solutions like electronic data capture and clinical trial management systems, and services such as consulting and support aiding in digital transformation. Geographically, North America has been dominant, but Asia-Pacific is rapidly growing due to improved healthcare facilities and R&D investments.
- Key players like Oracle, Medidata, and IQVIA are leading innovation in digital technologies for clinical trials, offering comprehensive software suites and advanced analytics solutions. Emerging players such as CliniOps and Veeva Systems are focusing on streamlining processes through specialized services like regulatory compliance and integrated data platforms.
- Pharma clinical trial digitization enhances operational efficiencies, patient engagement, and retention. Digital tools and health technologies improve recruitment rates, participant satisfaction, and data collection for more reliable study outcomes. Industry players are investing in AI, blockchain, and IoT to revolutionize drug development and enhance research reliability.
**Global Pharma Clinical Trial Digitization Market, By Services, Application, Themes, Country**
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Key Questions Answered with this Study
1) What makes Pharma Clinical Trial Digitization Market feasible for long term investment?
2) Know value chain areas where players can create value?
3) Teritorry that may see steep rise in CAGR & Y-O-Y growth?
4) What geographic region would have better demand for product/services?
5) What opportunity emerging territory would offer to established and new entrants in Pharma Clinical Trial Digitization Market?
6) Risk side analysis connected with service providers?
7) How influencing factors driving the demand of Pharma Clinical Trial Digitization in next few years?
8) What is the impact analysis of various factors in the Global Pharma Clinical Trial Digitization Market growth?
9) What strategies of big players help them acquire share in mature market?
10) How Technology and Customer-Centric Innovation is bringing big Change in Pharma Clinical Trial Digitization Market?
Browse Trending Reports:
Electric Impedance Tomography Market
Flexible Drum Liner Market
Fungicide Packaging Market
Secure Logistics Market
Ortho Pediatric Devices Market
Direct Current (DC) Motor Control Devices Market
Adams Oliver Syndrome Market
Antimicrobial Packaging Market
Astable Multivibrator Market
Cryptococcosis Market
Aarskog Syndrome Treatment Market
P2X7 Receptor Antagonists Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975